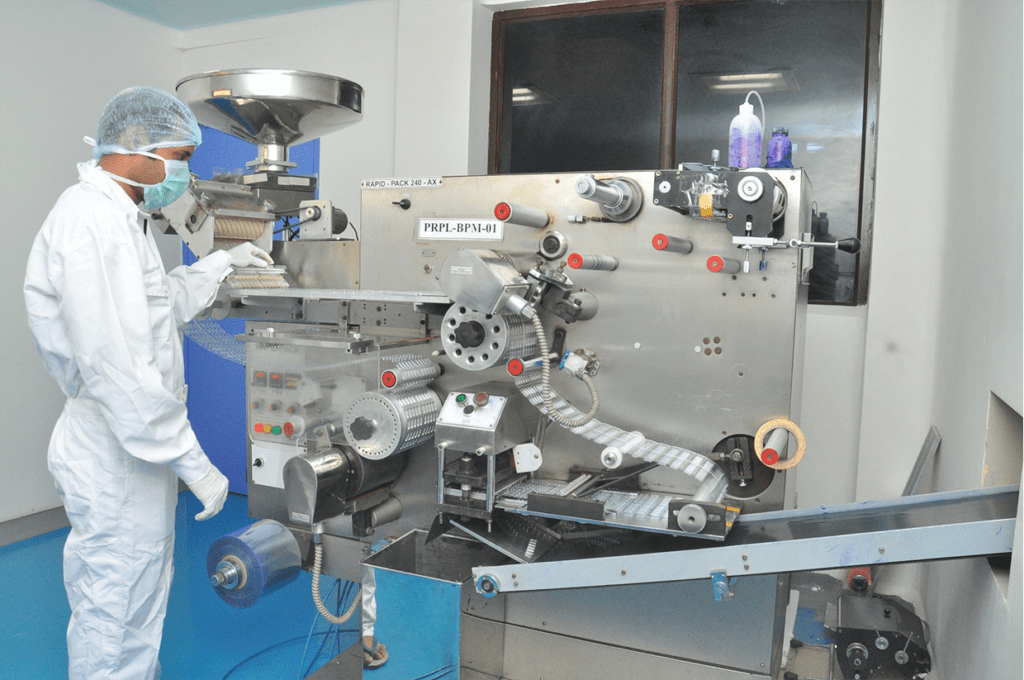
Manufacturing
Manufacturing in accordance with WHO-GMP regulations norms, Pilot Plants, Process development labs, In – Process QC labs and dedicated product processing areas are common features across every plant at Revinto. All manufacturing facilities are designed as per WHO-GMP guidelines.
Being one of the fastest growing pharmaceutical companies in India, we have always invested in building modern manufacturing facilities to increase our production capacities and meet the growing demand of our products. We have separate dedicated facilities to manufacture various dosage forms like tablets, hard and soft gelatin capsules, orally disintegrating films, syrups & suspensions, creams & ointments.
We have a highly skilled team of regulatory affairs specialists who are well versed with regulatory policies and procedures around the world. They have a wealth of experience in the timely filing of dossiers as well as handling regulatory queries from both authorities and customers.
The quintessentially state-of-the-art manufacturing infrastructure is truly the backbone of our company. Equipped with such modern facilities, our highly efficient and experienced production personnel are being able to cope up with the increasing demand of our products consistently. At the same time, the quality of the products is also being prioritized. So, the exemplary success of our company in terms of growth and profitability is the result of a confluence of knowledge and technology. Our manufacturing capabilities are perfectly attuned to our growth aspirations with continued investments for upgrading the manufacturing capacities.